Good Manufacturing Practices
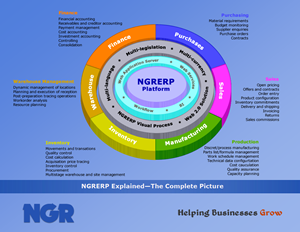
Good Manufacturing Practices (GMP) refers to an organization’s ability to ensure that products are consistently produced and controlled to appropriate quality standards in the production of foods, pharmaceutical products, and medical devices. GMP covers all aspects of the manufacturing process, product storage and transport, serial number tracking, and lot traceability.
NGRERP supports GMP through audit controls of transactions, electronic signatures, and version control with engineering change management of formulas and bills of materials. NGRERP also enables forward and backward lot traceability, shelf life management, and tracking and control of dangerous and restricted items. The overriding principles of GMP include designing quality into a product and controlling all aspects of the production process and associated material flow.